-
Vibrational Circular Dichroism of Adsorbed Molecules: BINAS on Gold Nanoparticles
C. Gautier and T. Bürgi
Journal of Physical Chemistry C, 114 (38) (2010), p15897-15902


DOI:10.1021/jp910800m | unige:14750 | Abstract | Article HTML | Article PDF
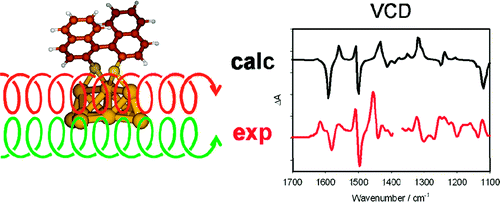
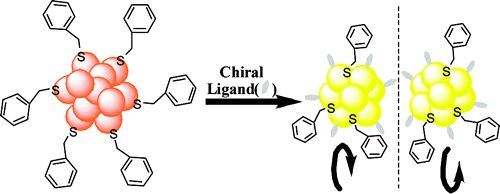
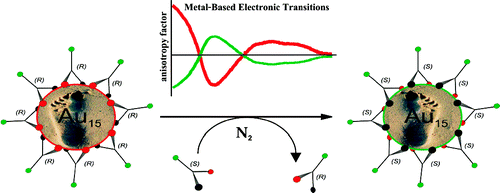
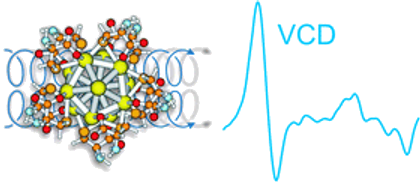
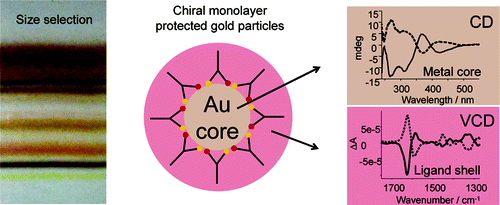
Vibrational circular dichroism (VCD) spectra of small size-selected gold nanoparticles covered by both enantiomers of 1,1′-binaphthyl-2,2′-dithiol (BINAS) were measured. VCD spectra of particles covered by the opposite enantiomers of BINAS show a mirror image relationship. The VCD spectrum of adsorbed BINAS is different from the one of free BINAS and its disulfide form, but it resembles more the dithiol form. Detailed analysis reveals that the angle between the two binaphthyl rings of BINAS is close to 90° for the adsorbed BINAS, similar to what is found for the free molecule. VCD spectra are quite insensitive to the particles size, in contrast to the electronic CD spectra, which change drastically as the particle size increases. This indicates that the vibrational characteristic is a local property. A model of BINAS adsorbed on a Au10 cluster was used to calculate VCD spectra. As for free BINAS and the disulfide the calculated spectrum of the adsorbed BINAS is in very good agreement with the measured one. This shows the potential of VCD spectroscopy to gain insight into the conformation of chiral molecules adsorbed on small metal particles.